Get a detailed overview of the possibilities and limits of a Distant Assessment/ Remote Audit. Both perspectives will be considered, that of the auditor and that of the auditee.
MHRA and FDA Joint Paper ‘Data Integrity in Global Clinical Trials’
The MHRA posted the following news on their website: https://mhrainspectorate.blog.gov.uk/2020/06/30/mhra-and-fda-joint-paper-data-integrity-in-global-clinical-trials/
It says that: “MHRA and FDA have produced a joint paper ‘Data Integrity in global Clinical Trials’ authored by those who presented at the event.”
If you click on the link below of the MHRA post you are forwarded to: https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1002/cpt.1794
There you can buy the mentioned joint paper – starts at 7 USD for 48 hours.
We give to that simply: No comment!
Let us only add something… both agencies are members of the PIC/S and ICH.
GMP Paper 4.0 – a retrofit solution
A lot of GMP regulated companies are currently in a transition period from a hybrid approach (paper-based / electronic data storage) to paperless processes. Such projects might be named to Industry 4.0 or digitalisation, paperless lab, LIMS etc.; or might be executed as part of a Data Integrity / Data Management program to reduce manual data inputs and transcriptions.
In reality it must be mentioned that GMP records, such as batch or laboratoy records might be a summary out of 20 to 60 print-outs (attached) = data sources = computerized systems. These systems and applications might be in place since many years and may not be ready for digitalisation / automated interfaces, but do fulfill technical and compliance requirements. So it might be better and more realistic to plan an intermediate step between the “classical” hybrid approach to full digitalisation and to define realistic timelines for the final implementation. During this “extended” hybrid approach solutions might be useful which could be defined as “retrofit” approach to existing systems, because the update or total replacement of such systems might take some time.
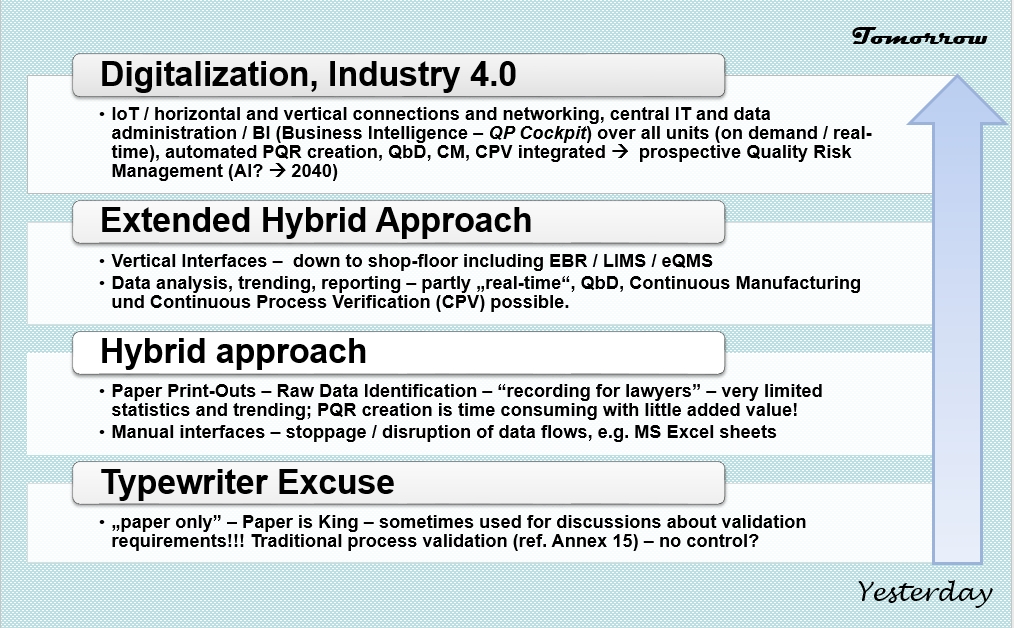
CCS has developed a retrofit solution regarding the records management approach for all quality, production and laboratory records, which is called GMP Paper 4.0. In addition creating print-outs will be required also in future for regulatory purposes, so it will be a solution for the transition period and afterwards.
But what is Paper 4.0 and how can it be used:
- Paper 1.0: Print-outs on simple office paper (format DIN A4 or Letter, 80 g/m², white)
- Paper 2.0: Print-outs on copy-proofed paper (anti-copy paper or copy-reactive paper with hidden message or watermarks)
- Paper 3.0: Print-outs on Paper 1.0 oder 2.0 with a QR code containing records meta-information to electronic storage, locations, authors, status etc.
- Paper 4.0: Print-outs including an NFC Sticker / Tag (on paper, as part of the paper)
Our GMP NFC Stickers for records are qualified, can’t be removed from the record (original or copy), are definitly unique by ID numbers during manufacturing and could be fully protected against data changes. Any device with NFC can read the information of the record, verify the authenticity of the print-out and all related meta information of the record (online connection required for verifications).
Our validated GMP Records Management system (RM Manager) is used for the registration, hand-over, control and administration of all GMP records, even if such records are created by existing systems not connected to a central database.
Please contact us at talk@comes-services.com for more information.
New PIC/S PI041 revision issued – now DRAFT 3
On 30 November 2018 PIC/S revised the current revision of PI 041-1 – DRAFT 3.
UPDATE 2021 – PIC/S PI 041 – Final Version – 1. July 2021
Here is the announcement from the PIC/S website (source)
Focused stakeholders consultation on revised draft PIC/S guidance on Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments
Geneva, 30 November 2018: a revised Draft PIC/S Guidance on Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments (PI 041-1 (Draft 3)) has been prepared by the PIC/S Working Group on Data Integrity, co-led by Australia / TGA and UK / MHRA.
The purpose of the guidance is to serve to outline the position an inspector would adopt during the inspection of GDP/GMP facilities and is designed to facilitate a harmonised approach to the inspection, including reporting in regards to data management and integrity. A first draft (PI 041-1 (Draft 2)) was published by PIC/S on a trial basis in August 2016. Following feedback received from PIC/S Participating Authorities during its 6-month implementation trial-period, the draft of this guidance has been updated and expanded by the Working Group.
The document (PI 041-1 (Draft 3)) is subject to a focused stakeholder consultation seeking substantive comments from trade and professional associations on specific questions relating to the proportionality, clarity and implementation of the guidance requirements. In parallel to this stakeholder consultation, the new draft will be applied by PIC/S Participating Authorities on a trial basis for a new implementation trial period.
The consultation period will last 3 months and run from 30 November 2018 to 28 February 2019.
To submit feedback, please provide feedback exclusively on the dedicated template available on the websites of the below associations and submit by e-mail with subject line “PIC/S Focused Public Consultation – Data Management and Integrity” to one of the following associations which will collect and compile responses. Stakeholders should only reply once.
- ECA (European Compliance Academy) Foundation:
send to: heimes@gmp-compliance.org- IFPMA (International Federation of Pharmaceutical Manufacturers & Associations):
send to: s.adam@ifpma.org- ISPE (International Society for Pharmaceutical Engineering):
send to: regulatorycomments@ispe.org- PDA (Parenteral Drug Association):
send to: tmorris@pda.orgTo download the consultation document (PI 041-1 (Draft 3)) please consult the page “Publications” or click on the link below:
End of announcement.
CCS is preparing a detailed review in January 2019 in order to provide substantive comments on the current DRAFT 3 version.
Read more – Download Center
New AGIT guidelines on computerized systems (GLP)
The Swiss Working Group of Information Technology (AGIT, Arbeitsgruppe Informationstechnologie) consists of representatives of the GLP Compliance Monitoring Units and of invited experts from the industry. Several documents related to information technology in a GLP environment (status of 31.01.2018) have been developed and can be downloaded (source: admin.ch) here:
Thanks to the AGIT group for this excellent source of information and sharing it to the public in English language.
Our latest article on LinkedIn – EC’s GMP Website
Read here our latest article on LinkedIn about the EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines website and presence.
Is our criticism justified and fair?
MHRA’s GXP data integrity guide published – March 2018
After the British agency MHRA has published three (3) different draft versions of the – MHRA GMP Data Integrity Definitions and Guidance for Industry – in January and March 2015 (GMP) and in July 2016 (Draft for consultation – now for GXP) the final “Medicines & Healthcare products Regulatory Agency (MHRA) ‘GXP’ Data Integrity Guidance and Definitions was published in March 2018.
Looking into the history of the document and content the major changes have been to replace and extend from “GMP” to GXP (covering GCP, GDP, GLP, GMP and GPvP). All this GXP areas had already requirements for data integrity or study integrity or similar for many years and are covered by European EMA regulations, directives and the EMA EudraLex Volumes or other regulations (e.g. chemical for GLP). The second change was the title “Guidance for Industry” to a “Guide“. Maybe the document type “Guidance for Industry” would remind you to US regulations and you are right. But in the EU such a document type in a regulatory structure and understanding is also not known. Maybe the roots of the first versions were coming from US or consultants or authors from this region. In general an own opinion might be better as to listen to any opinion-makers with commercial interests. Continue reading “MHRA’s GXP data integrity guide published – March 2018”
Upcoming Event: 27.11.2018 – How to validate Software Development tools used for GXP and MedDev?
After our successful event on 8. February 2018 we have planned to repeat it on the 27. November 2018 in the city of Stuttgart, Germany. Based on the input and feedback from the initial event we have improved several sessions and added a guest speaker.
Read more: Registration and Information page
If you have any questions please contact us at: talk@comes-services.com
GMP LOGFILE: Leitartikel – 16.01.2018
LOGFILE Nr. 02/2018 – Regulatorische Anforderungen an ein elektronisches Chargendokumentations- und Freigabesystem
Microsoft Azure GXP Guideline now published – reviewed by CCS
The goal of this GXP guidelines document is to provide life sciences organizations with a comprehensive toolset for using Microsoft Azure while adhering to industry best practices and applicable regulations. It identifies the shared responsibilities between Microsoft and its life sciences customers for meeting regulatory requirements, such as US-FDA 21 CFR Part 11 Electronic Records, Electronic Signatures (21 CFR Part 11), and EudraLex Volume 4 – Annex 11 Computerised Systems (EMA Annex 11).


